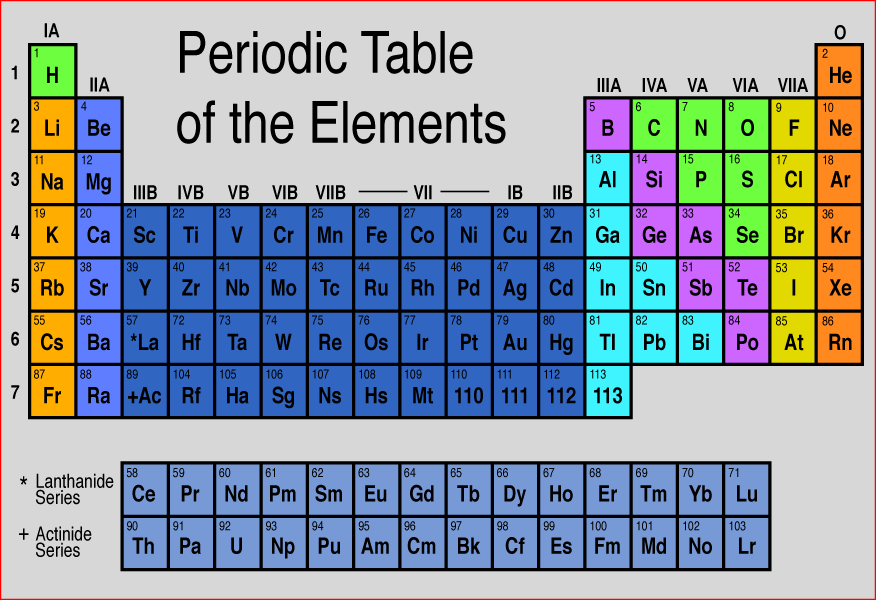
If your child has already learned about the parts of an atom (proton, neutron, and electron) they can understand the basics of the periodic table. From a basic periodic table, a young student can find: 1) the abbreviation for the element’s name, 2) how many protons an atom of the element has, 3) how many neutrons an average atom of the element has, 4) the number of electrons a neutral atom of the element has. Use the information below to teach your child the basics of using the periodic table. Then, turn the Periodic Table into a game. Give “clues” to a particular element and have your child use the clues to identify the element. You’ll find few examples of element clues at the end of this post to get you started.
Here’s an overview of periodic table basics:
 The one or two letter symbol in each box is an abbreviation for the name of the element. In the example on the right, the abbreviation for carbon is C.
The one or two letter symbol in each box is an abbreviation for the name of the element. In the example on the right, the abbreviation for carbon is C.
The number that is always above the symbol is the atomic number of the element. The atomic number gives the number of protons in one atom of that element.
The number under the symbol is the atomic mass. It is the average number of protons plus neutrons found in one atom of the element. You can use that number to find the number of neutrons in an average atom. Just subtract the number of protons from the rounded atomic number and you will have the average number of neutrons in one atom of the element.
Finally, in a neutral atom the number of electrons is always the same as the number of protons. So, the atomic number also gives the number of electrons in a neutral atom of the element.
Element clues:
1. Which element has 80 protons?
2. Which element has 16 neutrons and 16 electrons?
3. Which element’s name is abbreviated Fe?
Continue using the periodic table to make clues as long as the game holds the student’s interest. The more they become familiar with the periodic table now, the less intimidating it will be in upper level science classes!